Overview of distilling from 1909
The following are some of the most popular sources for fermenting alcohol: grapes, apricots, cherries, peaches, currents, gooseberries, raspberries, strawberries, figs, plums, bananas, carrots, turnips, beet-root, sweet corn, rice and other grains. As well as sugar-cane refuse, sorghum, and molasses.
Preparing Mash: When working with starchy materials one must first convert the starch into sugar. The first step in 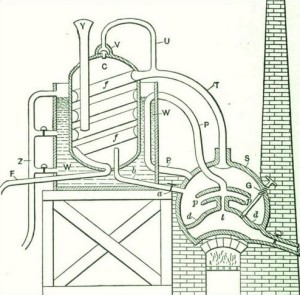 this process is the gelatinizing of the starch. This is done by heating it with water, or into a liquid mass by steaming it under high pressure. After this this is accomplished, the mash is then cooled. This is done in order to break up the gelatinized starch into a simpler sugar called “maltose” which the yeast can then processes into alcohol.
this process is the gelatinizing of the starch. This is done by heating it with water, or into a liquid mass by steaming it under high pressure. After this this is accomplished, the mash is then cooled. This is done in order to break up the gelatinized starch into a simpler sugar called “maltose” which the yeast can then processes into alcohol.
Fermentation: The maltose or sugar in the “mash” is now to be converted into alcohol. This is accomplished by fermentation, a process of decomposition which converts the sugar into carbonic acid and alcohol. Fermentation is started by yeast, which in the course of its life history produces a matter called zymogen which chemically acts in the sugar to split it up into carbonic acid gas and alcohol.
Yeast may be either “wild” or cultivated. If the mash is left to stand under proper condition the wild yeast spores in the air, will soon settle in the mash and begin to multiply. This method of fermentation is bad because other organisms antagonistic to proper fermentation. As a consequence, pure or cultivated yeast is alone used.
This yeast is cultivated from a mother bed in a special yeast mash and when ripened is mixed with the mash in their fermentation vat at a temperature between 50 and 86 degrees Feht. The yeast induces fermentation, converting the sugar of the mash into carbon dioxide which escapes, and alcohol which remains in the decomposed mash, or “beer” as it is termed in the United States.
It now remains to separate the alcohol from the water of the beer with which it is mixed. This is accomplished by distillation.
Mashing starchy materials: Production of alcohol for substances: Substance such as grape juice, fruit juice, sugar beets, cane sugar and molasses already contain fermentable sugar. We will now consider in more detail the preparation of mash from starch-containing substances.
Fermentation: Is an obscure and seemingly spontaneous change or decomposition which takes place in most vegetables and animal substances when exposed at ordinary temperatures to air and moisture. While fermentation broadly covers decay or purification, yet it is limited in ordinary use to the process of producing alcohol liquors from sacchariferous mashes.
Active fermentation is the process by which the sugar is processed into alcohol and carbon dioxide. The process continuing from 48 hours to several weeks according to the temperature, the amount of sugar present, and the nature and quantity of the ferments. Fermentation cannot occur at a temperature much below 40 degrees F., more above 140 degrees F. The limits of practical temperature, however, are 41 to 86 F. Brewer’s yeast is chiefly employed in spirit manufacturing.
The most striking phenomena of fermentation are the turbidity of the liquid, the rising of gas bubbles to the surface, and the increase in temperature, the disappearance of the sugar, the appearance of alcohol and the clearing of the liquid. At the end a slight scum is formed on the top of the liquid and a light colored deposit at the bottom. This deposit consists of yeast which is capable of exciting the vinous fermentation in other solution of sugar. The lower the temperature the slower the process, whole at a temperature above 86 degrees F, the vinous fermentation is liable to pass into other forms of fermentation to be hereafter considered.
Yeast:(click here to buy distilling yeast)
Yeast is a fungus, a mono-cellular organism, which under proper conditions propagates itself to an enormous extent. That are many races or varieties of yeast each having its peculiar methods of growth.
For our purposes we may divide the yeast races into two classes, wild yeast and cultivated yeast. Originally any of the yeast races were supposed to be good enough to effect fermentation but to day every effort is made to procure and use only those races which have the greatest power to decompose sugar. I was for this reason that the old distiller kept portions of his yeast over from one fermentation to the next. This was yeast whose action they understood and those abilities were proven. This yeast so kept was open, however, to the chance of contamination and yeast today is carefully selected and bred as in a strain of horses, or dogs, or plants.
After getting a portion of selected pure yeast for breeding purposes, it may be sowed, that is, propagated very carefully in a yeast mash, in sterilizing apparatus, where all chance of contamination by bacteria or wild yeast is avoided. Form this bed of mother yeast, or start yeast, the yeast for the successive yeast mashes is taken.
The preparation of the various varieties of yeast mashes is too lengthy to be set forth except in special treatises on the subject, but the ordinary method of yeasting is aa follows. Fig 5, which shows the apparatus, used in the yeasting and fermentation departments of a distillery, as installed by the Vulcan Copper Works, of Cincinnati. The yeast tubs are shown to the left of the illustration. They are each provided with the cooling coils and stirrers.
The yeast mash we will assume is composed of equal parts of barley malt and rye meal. Hot water at 166 degrees F, is first put into the mash tub. The rake or stirrers are then rotted and the meal run in slowly. The stirring is continued for twenty minutes after the meal is all in, during which the mash has become saccharified.
The mash is then allowed to stand for about twenty hours, and to grow sour by lactic fermentation. The lactic acid so produced protects the mother yeast from infection by suppressing wild yeast and bacteria. During this period great care is taken to prevent the temperature for the mash falling below 95 degrees F, and consequent butyric and acetous fermentation following. After it has so stood the sour mash is cooled by circulating water in the coils and stirring until it is reduced to from 59 to 68 F depending on whether the mash is thin or thick Start yeast during the cooling of the mash when at above 86 F is added and stirred in for the next twelve hours the yeast ferments and when a temperature of 84 F has been attained the mash is cooled to 65 F. at which temperature it is maintained until allowed to enter the fermenting tubs through the pipe leading thereto from the yeast tub.
There are four principal kinds of fermentation alcoholic acetous lactic and viscous.
Alcoholic Fermentation. This may be briefly described as follows The mash in the fermenting vat having been brought to the proper temperature the ferment is thrown in and the whole is well stirred together.
This is known as pitching. The proper pitching temperature varies with the method of fermentation adopted the length of the fermenting period the materials of the mash its thickness or attenuation. It must always be remembered that there is a great increase in the temperature of the beer during fermentation and that the temperature at its highest should never under any circumstances become greater than 86 F and with thick mashes that even a less heat is desirable. Therefore the pitching temperature should be such that the inevitable rise due to fermentation shall not carry the temperature to or beyond the maximum point desired for the particular mash being treated It is to accurately control the pitching temperature and the fermenting temperature that the fermenting tanks are provided with cooling appliances.
In about three hours’ time the commencement of the fermentation is announced by small bubbles of gas which appear on the surface of the vat and collect around the edges. As these increase in number the whole contents are gradually thrown into a state of motion resembling violent ebullition by the tumultuous disengagement of carbonic anhydride. The liquor rises in temperature and becomes covered with froth At this point the vat must be covered tightly the excess of gas finding an exit through holes in the lid care must now be taken to prevent the temperature from rising too high and also to prevent the action from becoming too energetic thereby causing the contents of the vat to overflow. In about twenty four hours the action begins to subside and the temperature falls to that of the surrounding atmosphere, An hour or two later the process is complete the bubbles disappear and the liquor which now possesses the characteristic odor and taste of alcohol settles out perfectly clear. The whole operation as here described usually occupies from forty eight to seventy two hours. The duration of the process is influenced of course by many circumstances chiefly by the bulk of the liquor its richness in sugar the quality of the ferment and the temperature.
Acetous Fermentation. This perplexing occurrence cannot be too carefully guarded against It results when the fermenting liquor is exposed to the air. When this is the case the liquor absorbs a portion of the oxygen which unites with the alcohol thus converting it into acetic acid as rapidly as it is formed. When acetous fermentation begins the liquor becomes turbid and a long stringy substance appears which after a time settles down to the bottom of the vat  It is then found that all the alcohol has been decomposed and that an equivalent quantity of acetous acid remains instead It has been discovered that the presence of a ferment and a temperature of 68 to 95 F are indispensable to acetous fermentation as well as contact with the atmosphere. Hence in order to prevent its occurrence it is necessary not only to exclude the air but also to guard against too high a temperature and the use of too much ferment. The latter invariably tends to excite acetous fermentation It should also be remarked that it is well to cleanse the vats and utensils carefully with lime water before using in order to neutralize any acid which they may contain for the least trace of acid in the vat has a tendency to accelerate the conversion of alcohol into vinegar. A variety of other circumstances are favorable to acetification such as the use of a stagnant or impure water, and the foul odors which arise from the vats stormy weather or thunder will also engender it.
It is then found that all the alcohol has been decomposed and that an equivalent quantity of acetous acid remains instead It has been discovered that the presence of a ferment and a temperature of 68 to 95 F are indispensable to acetous fermentation as well as contact with the atmosphere. Hence in order to prevent its occurrence it is necessary not only to exclude the air but also to guard against too high a temperature and the use of too much ferment. The latter invariably tends to excite acetous fermentation It should also be remarked that it is well to cleanse the vats and utensils carefully with lime water before using in order to neutralize any acid which they may contain for the least trace of acid in the vat has a tendency to accelerate the conversion of alcohol into vinegar. A variety of other circumstances are favorable to acetification such as the use of a stagnant or impure water, and the foul odors which arise from the vats stormy weather or thunder will also engender it.
Lactic Fermentation. Under the influence of lactic fermentation sugar and starch are converted into lactic acid. When it has once begun it develops rapidly and soon decomposes a large quantity of glucose but as it can proceed only in a neutral liquor the presence of the acid itself speedily checks its own formation. Then however another ferment is liable to act upon the lactic acid already formed converting it into butyric acid which is easily recognized by its odor of rank butter Carbonic anhydride and hydrogen are evolved by this reaction. The latter gas acts powerfully upon glucose converting it into a species of gum called mannite so that lactic fermentation in itself an intolerable nuisance becomes the source of a new and equally objectionable waste of sugar. It can be avoided only by keeping the vats thoroughly clean they should be washed with water acidulated with five per cent of sulphuric acid. An altered ferment or the use of too small a quantity will tend to bring it about.
The best preventives are thorough cleanliness and the use of good fresh yeast in the correct proportion.
Viscous Fermentation. This is usually the result of allowing the vats to stand too long before fermentation begins. It is characterized by the formation of viscous or mucilaginous matters which render the liquor turbid and by the evolution of carbonic anhydride and hydrogen gases the latter acting as in the case of lactic fermentation and converting the glucose into mannite Viscous fermentation may generally be attributed to the too feeble action of the ferment It occurs principally in the fermentation of white wines beer and beet juice or of other liquors containing much nitrogenous matter. It may be avoided by the same precautions as are indicated for the prevention of lactic fermentation.
Periods of Fermentation. The operation of fermentation may be conveniently divided into three equal periods.
The first or pre-fermentation period is that when the yeast mixed into the mash is growing the temperature should then be kept at about 63 to 68 F during which time the yeast is propagated. The growth of the yeast is manifested by the development of carbonic acid gas and by a slight motion of the mash When alcohol is produced to an extent of say five per cent the growth of the yeast stops.
The second period of chief fermentation then begins. Carbonic acid is freely developed and the sugar is converted into alcohol. The temperature at this time should not exceed 81.5 F. The second period of fermentation continues about 12 hours when the last period commences.
During the third period or after fermentation there is a lessening of the formation of carbonic acid and a lowering of the temperature. In this stage the mash is kept at a temperature of 77 to 81 F.
In order to conveniently regulate the temperature of the mash the vat may be provided with a copper worm at the bottom thereof through which cold water is forced. This however need only be used for thick mashes. There are also various kinds of movable coolers used for this purpose.
There are a number of different forms which fermentation may take. The insoluble constituents of the mash in the process of fermentation are forced to the surface and form what may be termed a cover. If the carbonic acid gas bubbles seldom break this cover it indicates that the conversion of the sugar into alcohol and carbonic acid is proceeding very slowly and imperfectly. If however the cover is swirling and seething and particularly if the cover is rising and falling with every now and then a discharge of gas it is an indication that the conversion is properly proceeding. Foaming of the mash is to be prevented as the froth or foam flows over the mash tank and considerable loss is sustained. It may be prevented by pouring a little hot lard into the vat or petroleum provided its odor will not interfere with the use of the alcohol when distilled.
Water is added in small quantities near the termination of the second period of fermentation. This dilutes the alcohol in the mash and lessens its percentage and thus the further growth of the yeast is permitted.
After fermentation the mash takes either the form of a thick diluted pulp or of a thin liquor. Again the reader is reminded that the mash after fermentation contains alcohol mixed with water and that the next step in the process distillation is necessary merely to separate the alcohol from the water.
There is always some loss in the process of fermentation in other words the actual production is below the theoretical amount due. Theoretically one pound of starch should yield 11.45 fluid ounces of alcohol. With a good result 88.3 per cent of this theoretical yield is obtained with an average result of 80.2 per cent and with a bad result only about 72.6 per cent or less.
Distilling Apparatus. The Apparatus employed in the process of distillation is called a still and is of almost infinite variety. A still may be any vessel which will hold and permit fermented wash or beer to be boiled therein and which will collect the vapors arising from the surface of the boiling liquid and transmit them to a condenser. The still may be either heated by the direct application of fire, or the liquid in the still raised to the boiling point by the 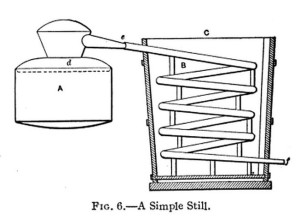 injection of steam. The steam or vapor rising from the boiling liquid must be cooled and condensed. This is done by leading it into tubes surrounded by cold water or the “cold mash.”
injection of steam. The steam or vapor rising from the boiling liquid must be cooled and condensed. This is done by leading it into tubes surrounded by cold water or the “cold mash.”
The very simplest form of still is shown in Fig 6, and consists of two essential parts the still or boiler A made of tinned copper the condenser C which may be made of metal or wood and the worm B made of a coil of tinned copper pipe.
The liquor is boiled in A and the vapors pass off into the worm B which is surrounded by the cold water of the condenser the distillate being drawn off at f.
The heated vapors passing through the worm B will soon heat up the water in C thereby retarding perfect condensation. To prevent this a cold water supply pipe may be connected to the bottom of C making a connection at the top of C for an over flow of the warmed up water. By this means the lowest part of the worm will be kept sufficiently cool to make a rapid condensation of the vapors.
The boiler A can be made in two parts; the upper part fitting into the lower part snugly at d. The pipe from the upper part fitting the worm snugly at e. This will enable the operator to thoroughly cleanse the boiler before putting in a new lot of liquor. The joints at e and d should be luted with dough formed by mixing the flour with a small portion of salt and moistening with water. This is thoroughly packed at the junctions of the parts to prevent the escape of steam or vapor. Fig 7 shows such a Still.
In an apparatus of this kind the vapors of alcohol and water are condensed together. But if instead of filling the condenser C with cold water it is kept at a temperature of 176 F. the greater part of the water vapor will be condensed while the alcohol which boils at 172.4 F passes through the coil uncondensed. If therefore the water be condensed 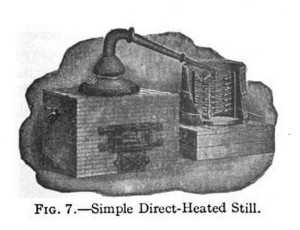 and collected separately in this manner and the alcoholic vapors be conducted into another cooler kept at temperature below 172.4 F the alcohol will be obtained in a much higher state of concentration than it would be by a process of simple distillation.
and collected separately in this manner and the alcoholic vapors be conducted into another cooler kept at temperature below 172.4 F the alcohol will be obtained in a much higher state of concentration than it would be by a process of simple distillation.
Supposing, again, that vapors containing but a small quantity of alcohol are brought into contact with an alcoholic liquid of lower temperature than the vapors themselves and in very small quantity the vapor of water will be partly condensed so that the remainder will be richer in alcohol than it was previously. But the water in condensing converts into vapor a portion of the spirit contained in the liquid interposed so that the uncondensed vapors passing away are still further enriched by this means. Here, then, are the results obtained; the alcoholic vapors are strengthened, firstly, by the removal of a portion of the water wherewith they were mixed; and then by the admixture with them of the vaporized spirit placed in the condenser. By the employment of some such method as this; a very satisfactory yield of spirit may be obtained both with regard to quality, as it is extremely concentrated, and to the cost of production, since the simple condensation of the water is made use of to convert the spirit into vapor without the necessity of having recourse to fuel. The construction of every variety of distilling apparatus now in use is based upon the above principles.
A sectional view of another simple form of still is shown in Fig 8; V is a wooden vat having a tight fitting cover a, through the center of which a hole has been cut. The wide end of a goose neck of copper pipe g is securely fitted over this aperture, the smaller end of this pipe passes through the cover of the retort R extending nearly to the bottom; f 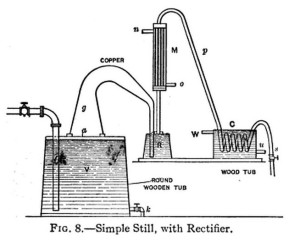 is the steam supply pipe from boiler M the rectifier consisting of a cylindrical copper vessel containing a number of small vertical pipes surrounded by a cold water jacket o the inlet for the cold water which circulates around these small pipes discharging at n the pipes in M have a common connection to a pipe p which connects the rectifier with coil in cooler C s is a pipe to the receptacle for receiving the distillate; u cold water supply pipe to cooler, and W discharge for warmed up water, k discharge for refuse wash in vat V.
is the steam supply pipe from boiler M the rectifier consisting of a cylindrical copper vessel containing a number of small vertical pipes surrounded by a cold water jacket o the inlet for the cold water which circulates around these small pipes discharging at n the pipes in M have a common connection to a pipe p which connects the rectifier with coil in cooler C s is a pipe to the receptacle for receiving the distillate; u cold water supply pipe to cooler, and W discharge for warmed up water, k discharge for refuse wash in vat V.
The operation is as follows: The vat V is nearly filled with fermented mash and retort R with weak distillate from a previous operation. Steam is then turned into the pipe discharging near the bottom of the vat V and working up through the mash. This heats up the mash and the vapors escape up g over into R where they warm up the weak distillate. The vapors thus enriched rise into M, where a good percentage of the water vapor is distilled, that is, condensed by the cold water surrounding the small pipes. The vapor then passes over through p into the coil where it is liquefied and from whence it passes by pipe s into the receiver. The cold water for cooling both M and C can be turned on as soon as the apparatus has become thoroughly heated up.
The stills in use to-day in many parts of the South for the production of whiskey are quite as simple as those above described, and some for the making of “moonshine” liquor are more so.
The first distilling apparatus for the production of strong alcohol on an industrial scale was invented by Edward Adam, in the year 1801. The arrangement is shown in Fig 9, in which A is a still to contain the liquor placed over a suitable heater. The vapors were conducted by a tube into the egg shaped vessel B, the tube reaching nearly to the bottom; they then passed out by another tube into a second egg C; then, in some cases, into a third not shown in the figure, and finally into the worm D, and through a cock at G into the receiver. The liquor condensed in the first egg is stronger than that in the still while that found in the second and third is stronger than either. The spirit which is condensed at the bottom of the worm is of a very high degree of strength. At the bottom of each of the eggs, there is a tube connected with the still, by which the concentrated liquors may be run back into A for redistillation after the refuse liquor from the first distillation has been run off.
One thing that isn’t mentioned in this article is that you will need an alcohol hygrometer in order to make your cuts. Cuts are where you decide what you want to keep, and what you want to through out. You can make your cuts on taste, but it’s a skill that takes time. It is also nice to be aware of the alcohol content of your spirits before you start the aging and bottling process. You should be able to find an alcohol hydrometer for less than $30.




As you continue to walk, the plantar fascia will get stretched out and ‘warms up’ and then the pain improves.